
Publisher:
Bonnie King
CONTACT:
Newsroom@Salem-news.com
Advertising:
Adsales@Salem-news.com

~Truth~
~Justice~
~Peace~
TJP
Sep-19-2021 22:03

 TweetFollow @OregonNews
TweetFollow @OregonNews
Rappaportupdated
Marianne Skolek-Perez Salem-News.com Investigative Reporter"It's time for impunity to end with the FDA": PART 1
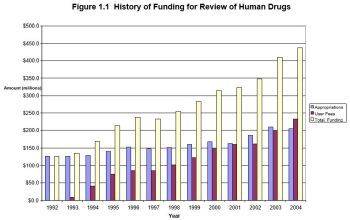 Source: FDA report |
(MYRTLE BEACH, SC) - In my 19 continuous years of exposing the FDA and their "pay to play" tactics with the pharmaceutical industry, I have had many articles published calling it as it is -- "the root of the opioid epidemic is at the top" with the FDA.
I will be running a series of these articles from the past naming the offenders in the FDA as well as the pharmaceutical industry. It will be aptly entitled "It's time for impunity to end with the FDA."
I hope the series helps those who need to be educated as to the meaning of the word "criminals." Here is an article I wrote in November 2014 which was published in Salem-News.com regarding Bob Rappaport, MD, Division Director of Anesthesia, Analgesia and Addiction Products at the FDA.
What if the head of the division of the FDA responsible for opioids being approved is not a "watch dog" to the American people, but rather is a "lap dog" to the pharmaceutical industry?
The retirement of Dr. Rappaport was announced by the FDA at the end of September. Sharon Hertz, MD was named "Acting Division Director" filling Rappaport's position.
Strange though, it is now into November and emails sent to Rappaport, do not direct the sender to Dr. Hertz with her email address. Rather, the following email is returned to the sender:
bob.rappaport@fda.hhs.gov
The e-mail address you entered couldn't be found.
Please check the recipient's e-mail address and try to resend the message.
If the problem continues, please contact your helpdesk.
One of Dr. Rappaport's biggest contributions to the American people before the FDA announced his retirement was an opioid called Zohydro ER.
Zohydro ER will be the first hydrocodone-only opioid in doses of 5 to 10 times more heroin-like narcotic than Vicodin. The FDA disregarded their own Advisory Committee who voted 11-2 not to approve the opioid because of the Committee's concerns about the potential impact on public health.
How could the FDA approve Zohydro ER when there is no abuse deterrent built into the drug so when it is crushed, chewed or mixed with alcohol -- the high probability of death exists? Didn't the FDA violate their own directive that opioids would only be approved by them if there were a built-in abuse preventative in the drug? Dr. Rappaport was quoted as saying the FDA did not want to be seen as "punishing this company (Zogenix) and this drug (Zohydro ER) because of the sins of other companies and their product."
After Zohydro ER was approved by the FDA, Purdue Pharma, of OxyContin fame, prepared to have their equivalent of Zohydro ER approved by the FDA.
Purdue Pharma has been granted Priority Review status by the FDA for its New Drug Application (NDA) for extended-release hydrocodone bitartrate, called Hysingla/ER.
Hysingla/ER, although crush-resistant, may be more dangerous than Zohydro ER because it is a once-a-day pill, packing more than twice as much hydrocodone in a maximum dose of 120 mgs compared to 50 mgs for Zohydro ER.
So we have two pharmaceutical companies competing for long term chronic pain treatment in an opioid nicknamed "heroin in a pill."
How could these two competitors profit individually in pushing Zohydro ER and Hysingla/ER to the medical profession? Easy.
This past week the makers of Zohydro ER and Hysingla/ER exchanged waivers of regulatory exclusivity for extended-release products which includes $10 million in payments and potential sales royalties from Purdue Pharma.
A copy of the filing to the "FDA" is shown here: http://www.sec.gov/Archives/edgar/data/1375151/000137515114000025/ex104-2014930.htm.
As a bit of a background on the FDA, it was founded in 1906 and was supported by the U.S. Treasury. In 1992, this funding changed to new drug applications funding coming from the pharmaceutical industry. In other words, if you want your drug reviewed by the FDA -- the pharmaceutical industry is the bank.
In October 2013, John Fauber wrote an article for the Milwaukee-Wisconsin Journal Sentinel about a "pay to play" with the FDA by the pharmaceutical industry.
The link to his article is here: http://www.jsonline.com/watchdog/watchdogreports/emails-point-to-troubling-relationship-between-drug-firms-regulators-b99113286z1-226692641.html
Here are some highlights of Fauber's writing:
- Since 2002 drug companies have paid up to $35,000 each to send a representative to meetings of an organization called IMMPACT, where they could discuss clinical trial testing procedures with officials from the FDA and other government agencies. The goal of IMMPACT is to improve the design of clinical trials conducted to develop new pain treatments.
It became known as a "pay-for-play arrangement where pharmaceutical companies could buy their way into "invitation only" meetings with the FDA and in essence affect FDA pain drug policy. Here is IMMPACT's website http://www.immpact.org/. The two heads of IMMPACT are Dennis C. Turk, PhD and Robert H. Dworkin, PhD.
Back to the esteemed Dr. Rappaport's "retirement." The FDA proudly announced that Rappaport would be the recipient of the John and Emma Bonica Public Service Award honoring outstanding contributions by an individual or an organization to the field of pain through public education, dissemination of information, public service, or other efforts to further knowledge about pain."
Dr. Rappaport will receive this award at the 2015 annual American Pain Society meeting to be held in Palm Springs, California on May 13 - 16, 2015.
The American Pain Society is under U.S. Senate investigation for their involvement in the opioid epidemic reaching epic proportions in the U.S.
Here is a list of some of the past recipients of this "esteemed" award:
2014 Award Recipients
- Robert H. Dworkin, PhD
- Dennis C. Turk, PhD
- Robert Kerns, PhD, 2010
- Perry Fine, MD, 2008
- Scott M. Fishman, MD, 2004
- David E. Joranson, 1999
- http://www.salem-news.com/articles/november172013/dod-pay-play-ms.php
http://www.salem-news.com/articles/november252013/pain-profits-ms.php
http://www.salem-news.com/articles/august122012/perry-fine-folo-ms.php
http://www.salem-news.com/articles/september092012/prescription-disaster-ms.php
http://www.salem-news.com/articles/april222011/pain-studies-ms.php
Somehow I don't think Dr. Rappaport will be spending his "retirement" gardening. Time will tell if he will have a corner office in the pharmaceutical industry -- wearing his gold watch from the FDA.
Articles for September 19, 2021 |






Terms of Service | Privacy Policy
All comments and messages are approved by people and self promotional links or unacceptable comments are denied.
[Return to Top]
©2025 Salem-News.com. All opinions expressed in this article are those of the author and do not necessarily reflect those of Salem-News.com.