
Publisher:
Bonnie King
CONTACT:
Newsroom@Salem-news.com
Advertising:
Adsales@Salem-news.com

~Truth~
~Justice~
~Peace~
TJP
Oct-24-2016 21:35

 TweetFollow @OregonNews
TweetFollow @OregonNews
Is Janet Woodcock, MD, Director of FDA Guilty of Medical Malpractice?
Marianne Skolek-Perez, Salem-News.com Investigative ReporterHow many lives could have been saved if she had heeded AG Blumenthal's petition?
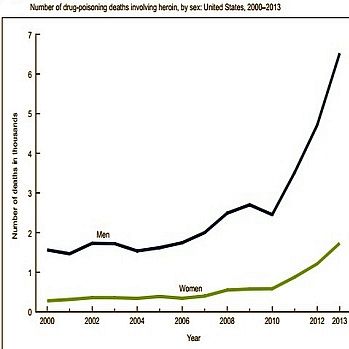 In 2000-2013 the rate for heroin-related overdose deaths was highest among adults aged 25-44. |
(MYRTLE BEACH, S.C.) -  Janet Woodcock, MD, is the director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA). The center makes sure that safe and effective drugs are available to improve the health of people in the United States.
Janet Woodcock, MD, is the director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA). The center makes sure that safe and effective drugs are available to improve the health of people in the United States.
In view of the prescription opioid epidemic evolving into a Tsunami of deaths and addictions, did Dr. Woodcock contribute to the volcanic eruption of the epidemic by violating rules of the FDA?
On January 24, 2004 then Attorney General (AG) Richard Blumenthal of Connecticut (now US Senator of Connecticut) submitted a Citizen Petition (CP) to the FDA entitled "Petition to Require Purdue Pharma L.P. to Revise the Labeling of OxyContin Tablets to Strengthen Warnings of the Greater Potential for Developing Side Effects and Adverse Drug Reactions Due to Prescribing Dosing Frequencies in Excess of the Recommended Guidelines".
AG Blumenthal's Citizen Petition is linked below. (The definition of a Citizen Petition off the FDA website -- including the time frame for response to the Citizen Petition by the FDA -- is shown below this article).
Although the FDA has two conflicting dates on their website as to the time frame to respond to a Citizen Petition of 5 months or 6 months, Janet Woodcock, MD did not respond to Blumenthal's 2004 CP until 2008.
Way beyond the 5 to 6 months the FDA is required to respond. Provided below is a link to Woodcock's response to Blumenthal -- all 18 pages of it.
SEE: https://www.scribd.com/document/328752805/Blumenthal-Cp-Woodcock
One of the comments Dr. Woodcock stated in her reply was that ".....although higher total daily dosages were associated with fatal outcomes, it cannot be concluded that the higher total daily dosages are causally associated with fatalities given the other variables have not been measured."
Further she writes "...there is no maximum dose for opioids."
Recently the Center for Disease Control (CDC) recommendations on prescribing opioids for chronic pain stated as follows "Benefits of high-dose opioids for chronic pain are not established."
The complete opposite of Woodcock's 2008 quotation of "no maximum dose".
So, answer me this Dr. Woodcock -- how many lives could have been saved when AG Blumenthal sent the FDA his Citizen Petition in 2004 if you had acted on it in a timely and scientific manner? What do you think? Hundreds? Thousands? Tens of Thousands? Do you believe that you are responsible for this out of control prescription opioid epidemic by your inaction? Or worse, could you be guilty of medical malpractice?
In Blumenthal's CP to the FDA, he references an interview he conducted with a woman in Iowa. She agreed to have her name used in his petition.
In 1999, the woman's physician prescribed her OxyContin 20 mgs twice a day. She complained to her doctor of many side effects and he treated what she called "addiction" to OxyContin by increasing her dosage.
In May 2002 acting on the advice of her orthopedic surgeon, she attempted "to wean herself from the drug." This attempt was short-lived and in September 2002, she voluntarily admitted herself into a drug treatment facility in Illinois where she spent two and one half days before leaving the program.
Later that month she spent one week going "cold turkey" in the basement of her parents' home before she was finally successful in coming off OxyContin.
I know this story first hand Dr. Woodcock, because I put this woman who was "addicted" to OxyContin in contact with AG Blumenthal and I knew her story quite well. So what I would like to know from you, Dr. Woodcock, is this:
How could you say in your September 9, 2008 reply to AG Blumenthal's CP -- almost 5 years after the deadline indicated on the FDA website to respond to a Citizen Petition -- that the woman referenced in the CP who had been interviewed by Blumenthal "ultimately weaned herself from the medication."
Really? Maybe you should look up the medical definition of "weaning". Going cold turkey on a basement floor for a week is not weaning. We both know that.
As a medical doctor, how could you state how this woman was treated or ceased to take OxyContin? You never interviewed her or examined her. Careless on your part Dr. Woodcock or a clear case of medical malpractice?
Citizen Petition
________________________________________________ Date:____________ The undersigned submits this petition under __ (relevant statutory sections, if known) of the __ (Federal Food, Drug, and Cosmetic Act or the Public Health Service Act or any other statutory provision for which authority has been delegated to the Commissioner of Food and Drugs) to request the Commissioner of Food and Drugs to__ (issue, amend, or revoke a regulation or order or take or refrain from taking any other form of administrative action).Off the FDA website - Section 505(q)(1)(F) governs the timeframe for final Agency action on a petition. Under this provision, FDA shall take final Agency action on a petition not later than 150 days after the date on which the petition is submitted. The 150-day period is not to be extended for any reason, including any determination made under section 505(q)(1)(A) regarding delay of approval of an application, the submission of comments or supplemental information, or the consent of the petitioner.
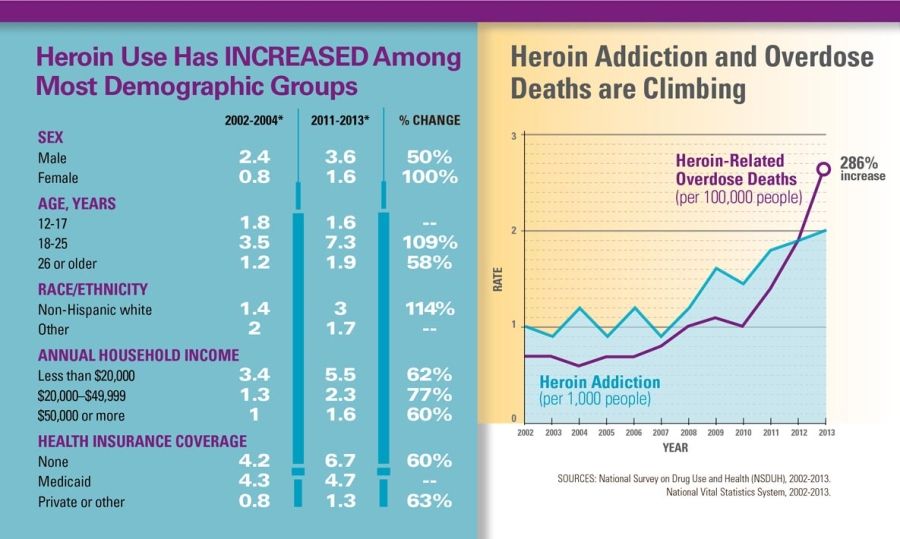
Next in a series: How Dr. Woodcock's statements in her 2008 reply to AG Blumenthal's Citizen Petition furthered the prescription opioid epidemic leading to an unprecedented rise in heroin deaths.
SEE ALSO:
Citizen Petition by AG Blumenthal
FDA-Code of Federal Regulations Title 21
Articles for October 24, 2016 | Articles for October 25, 2016
Salem-News.com:





Terms of Service | Privacy Policy
All comments and messages are approved by people and self promotional links or unacceptable comments are denied.
[Return to Top]
©2025 Salem-News.com. All opinions expressed in this article are those of the author and do not necessarily reflect those of Salem-News.com.