
Publisher:
Bonnie King
CONTACT:
Newsroom@Salem-news.com
Advertising:
Adsales@Salem-news.com

~Truth~
~Justice~
~Peace~
TJP
Oct-06-2021 15:02

 TweetFollow @OregonNews
TweetFollow @OregonNews
It's Time for Impunity to End with the FDA: PART 3
Marianne Skolek-Perez Salem-News.com Investigative ReporterPart 3 in my series of the FDA being held accountable for conflicts of interest with pharma
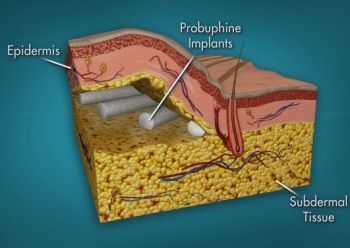 An implanted rod about the size of a matchstick delivers low-level buprenorphine for six months. Image: livenewscentral.com |
(MYRTLE BEACH, SC) - In June 2016, I wrote the below article for publication in Salem-News.com. It focused on the first buprenorphine implant for treatment of opioid dependence marketed by San Francisco-based Titan Pharmaceuticals Inc. and approved by the FDA on May 26, 2016.
In December 2015, Renaissance Technologies, LLC, a leading hedge fund institution, purchased 61,913 shares of Titan Pharmaceuticals.
Renaissance Technologies Co-Chief Executive Officer and Co-President and Director was Peter Fitzhugh Brown. Mr. Brown was a hedge funder and the husband of former FDA Commissioner, Margaret Hamburg, MD. Dr. Hamburg retired as commissioner of the FDA in March 2015.
Was there any investigation into a conflict of interest with Margaret Hamburg, Titan Pharmaceuticals, Peter Fitzhugh Brown and Renaissance Technologies?
Is there any oversight into Janet Woodcock, MD, current acting FDA Commissioner and her potential conflicts of interest with pharma and their COVID vaccines? Stay tuned.
Jun-14-2016
Is the FDA Involved in Criminal Activity with Insider Trading?
Marianne Skolek-Perez, Salem-News.com Investigative ReporterHere is an excerpt from a recent FDA News Release (link to release at end of article).
FDA approves first buprenorphine implant for treatment of opioid dependence.
Expanded use and availability of medication-assisted treatment is a top priority of federal effort to combat opioid epidemicFor Immediate Release May 26, 2016
"The U.S. Food and Drug Administration today approved Probuphine, the first buprenorphine implant for the maintenance treatment of opioid dependence. Probuphine is designed to provide a constant, low-level dose of buprenorphine for six months in patients who are already stable on low-to-moderate doses of other forms of buprenorphine, as part of a complete treatment program.
"Until today, buprenorphine for the treatment of opioid dependence was only approved as a pill or a film placed under the tongue or on the inside of a person’s cheek until it dissolved.
"While effective, a pill or film may be lost, forgotten or stolen. However, as an implant, Probuphine provides a new treatment option for people in recovery who may value the unique benefits of a six-month implant compared to other forms of buprenorphine, such as the possibility of improved patient convenience from not needing to take medication on a daily basis.
"An independent FDA advisory committee supported the approval of Probuphine in a meeting held earlier this year.
"Opioid abuse and addiction have taken a devastating toll on American families. We must do everything we can to make new, innovative treatment options available that can help patients regain control over their lives," said FDA Commissioner Robert M. Califf, M.D.
"Today’s approval provides the first-ever implantable option to support patients’ efforts to maintain treatment as part of their overall recovery program.”
Probuphine is marketed by San Francisco-based Titan Pharmaceuticals Inc.
**END**
Any word on who the members of the "independent" advisory committee were? That was not a part of the FDA press release.
Interesting that this committee approved Probuphine at the beginning of 2016.
Even more interesting is the fact that on December 15, 2015 one of the top institutional holders purchased 61,913 shares of Titan Pharmaceuticals. The name of the hedge fund institution making the purchase was Renaissance Technologies, LLC.
I have written about Renaissance Technologies (link to article "Is FDA's Margaret Hamburg, MD Contributing to the U.S. Heroin and Opioid Epidemic by Living in an Ivory Tower?") and its Co-Chief Executive Officer and Co-President and Director - Peter Fitzhugh Brown.
Mr. Brown is a hedge funder and the husband of former FDA Commissioner, Margaret Hamburg, MD. Dr. Hamburg retired as commissioner in March 2015.
Before Dr. Hamburg was confirmed as Commissioner of the FDA in 2009, she and her husband, Peter Fitzhugh Brown had to divest themselves of several hedge-fund holdings as well as some of Mr. Brown's inherited drug-company stocks so Dr. Hamburg could take the post as the nation's top food and drug regulator.
The couple's assets were valued at between $21 million and $40 million.
Hamburg's husband had to sell his pharmaceutical stocks in Abbott Laboratories, Johnson & Johnson, Merck and pharmacy-benefits manager Medco Health Solutions.
Abbott Laboratories promoted OxyContin, or "hillbilly heroin" to anesthesiologists, surgeons, emergency physicians and pain management teams in hospitals and surgery centers throughout the country beginning in 1996 -- while Purdue Pharma marketed OxyContin to all other physicians.
Medco Health Solutions is a pharmacy benefits managing company that handles drug benefits for over 60 million people.
Did Hamburg and her wealthy hedge fund husband, in fact, divest themselves of stocks in order for her to become the head of the FDA? Or was it an opportunity leading to insider trading during her tenure and after her retirement?
So who is doing the insider trading within the FDA, or is Dr. Hamburg's hedge fund husband just clairvoyant in what stocks to purchase for profit as he did with Titan Pharmaceuticals?
Criminal activity inside the FDA? You decide.
- salem-news.com/articles/april132014/hamburg-addiction-ms.php
- globalnewscentre.com/fda-commissioner-margaret-hamburg
- salem-news.com/articles/september022014/pharma-crimes-ms.php
- The FDA press release on buprenorphine implant in its entirety:
fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm503719.htm
Is the FDA Involved in Criminal Activity with Insider Trading? - Salem-News.com
Articles for October 6, 2021 |





Quick Links
DINING
Willamette UniversityGoudy Commons Cafe
Dine on the Queen
Willamette Queen Sternwheeler
MUST SEE SALEM
Oregon Capitol ToursCapitol History Gateway
Willamette River Ride
Willamette Queen Sternwheeler
Historic Home Tours:
Deepwood Museum
The Bush House
Gaiety Hollow Garden
AUCTIONS - APPRAISALS
Auction Masters & AppraisalsCONSTRUCTION SERVICES
Roofing and ContractingSheridan, Ore.
ONLINE SHOPPING
Special Occasion DressesAdvertise with Salem-News
Contact:AdSales@Salem-News.com
Terms of Service | Privacy Policy
All comments and messages are approved by people and self promotional links or unacceptable comments are denied.
[Return to Top]
©2025 Salem-News.com. All opinions expressed in this article are those of the author and do not necessarily reflect those of Salem-News.com.